The AMPLATZER PFO Occluder is indicated for percutaneous transcatheter closure of a patent foramen ovalePFO to reduce the risk of recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have had a cryptogenic stroke due to a presumed paradoxical embolism as determined by a neurologist and cardiologist following an. CLOSURE WITH THE AMPLATZER PFO OCCLUDER.
 Patent Foramen Ovale Pfo Closure Procedure Indications
Patent Foramen Ovale Pfo Closure Procedure Indications
It is used to treat patie.

Amplatzer pfo closure. The Amplatzer PFO Occluder consists of two nitinol wire mesh discs that can be placed in your heart to close the PFO through a minimally invasive catheter-based technique. The PFO occluder is designed to stop the blood flow and potential clots through the PFO. The device consists of two circular wire-mesh discs covered in a medical fabric that sandwich together to close the PFO between the two upper chambers in your heart the left atrium and right atrium.
2122 Interestingly device disc sizes of 30 mm and greater showed a. The earliest randomised trials of PFO closure Evaluation of the STARFlex Closure System in Patients with a Stroke andor Transient Ischemic Attack due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale CLOSURE I and Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism PC Trial did not demonstrate the superiority of closure. Patent Foramen Ovale Closure Amplatzer PFO Occluder.
24 Thus the Amplatzer attains closure results comparable to surgical PFO closure. Small PFO No ASA Routine 18 18 mm 8 French 8 French 9 French 9 French 10 French 18 25 mm 8 French 28 35 mm 9 French case Enormous ASA Very thick septum secundum Double cribriform 2525 30303535 4040 mm F. The Amplatzer PFO Occluder is indicated for percutaneous transcatheter closure of a patent foramen ovale PFO to reduce the risk of recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have had a cryptogenic stroke due to a presumed paradoxical embolism as determined.
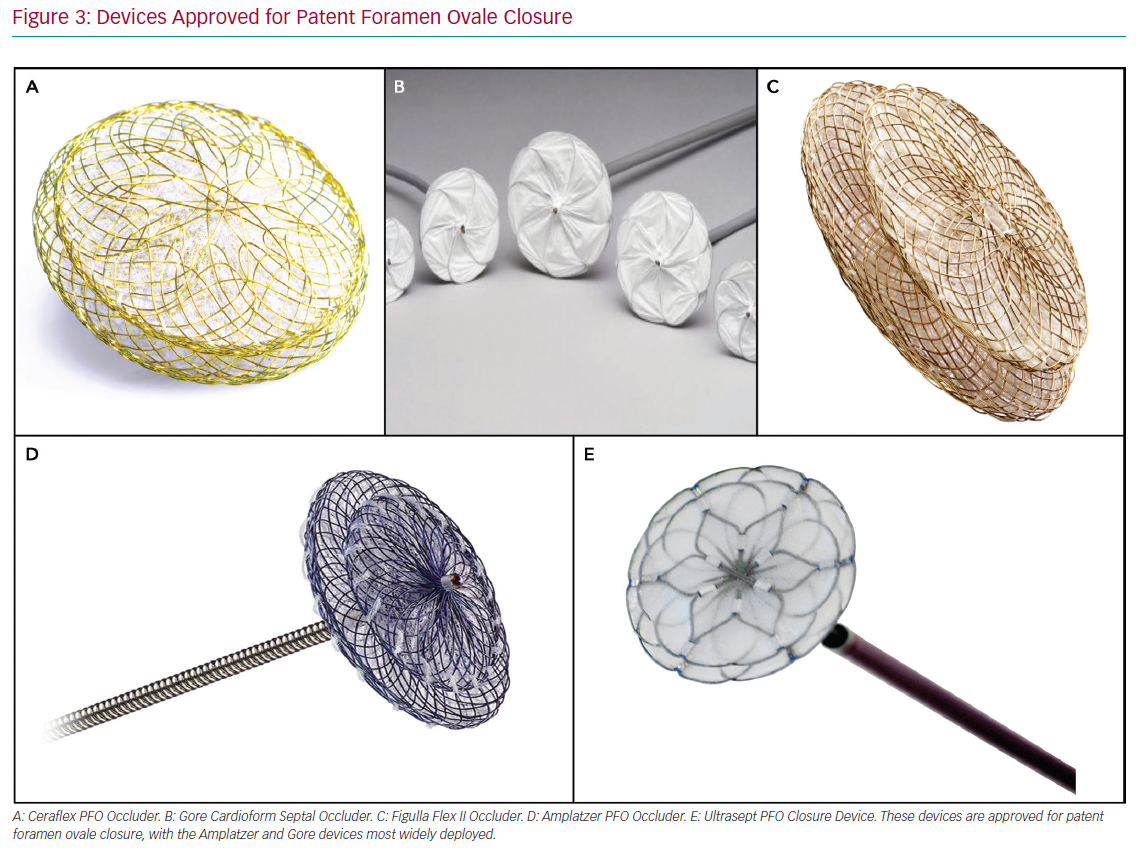
However closure of a patent foramen ovale with the Amplatzer PFO Occluder was superior to medical therapy alone in the prespecified per-protocol and. Family of Amplatzer PFO occluders. Transcatheter PFO closure in the United States has endured a long journey from the closure of ASDs in dogs in the 1940s to the first 2 FDAapproved PFO closure devices in the United States on October 28 2016 Amplatzer PFO Occluder and on March 30 2018 Gore Cardioform Septal Occluder.
The AMPLATZER PFO Occluder is intended for percutaneous transcatheter closure of a patent foramen ovale PFO to prevent recurrent ischemic stroke in patients who have had a cryptogenic stroke. An Amplatzer PFO occluder insert on September in Switzerland. Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism8 and RESPECT Randomized Evaluation of Recur-rent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment9 failed to demonstrate the superiority of PFO closure over antithrombotic therapy.
AMPLATZER PFO OCCLUDER INDICATION FOR USE The AMPLATZER PFO Occluder is indicated for percutaneous transcatheter closure of a patent foramen ovale PFO to reduce the risk of recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have had a cryptogenic stroke due to a presumed paradoxical embolism as determined. The Amplatzer PFO Occluder is a device specifically designed to stop blood flow through a PFO reducing your risk of a recurring cryptogenic stroke. The device consists of two circular wire -mesh discs.
The Amplatzer PFO Occluder is a device that can be placed in your heart to close the PFO. Gore and Associates Flagstaff Arizona. The Amplatzer device achieved complete PFO closure in 47 of 50 patients with only a minimal or moderate residual shunt deemed insignificant in three patients which corresponds to the rate observed by other investigators.
It is placed through a minimally invasive catheter-based technique. In the major study to evaluate the safety and effectiveness of the Amplatzer PFO Occluder most patients who were treated with the device also took. INDICATIONS AND USAGE The AMPLATZER PFO Occluder is indicated for percutaneous transcatheter closure of a patent foramen ovale PFO to reduce the risk of recurrent ischemic stroke in patients predominantly between the ages of 18 and 60 years who have had a cryptogenic stroke due to a presumed paradoxical embolism as determined by a neurologist.
The AMPLATZER TM PFO Occluder is a device specifically designed to stop blood flow through a PFO. Outside of the United States these and similar devices have been available for over 25 years. The Amplatzer PFO Occluder is the leading PFO closure device developed specifically for occlusion of a patent foramen ovale PFO.
The Amplatzer PFO Occluder is a device that can be placed in the heart to close a Patent Foramen Ovale using minimally-invasive methods. In October 2016 the FDA approved the use of the Amplatzer PFO Occluder Abbott Chicago Illinois device for PFO closure and in March 2018 use of the Gore Cardioform Septal Occluder WL. AMPLATZER TM PFO Occluder Device Description.

