The most common adverse reactions are somnolence headache nasal discomfort throat irritation and rhinorrhea10. Nayzilam is a federal controlled substance C-IV because it can be abused or lead to dependence.
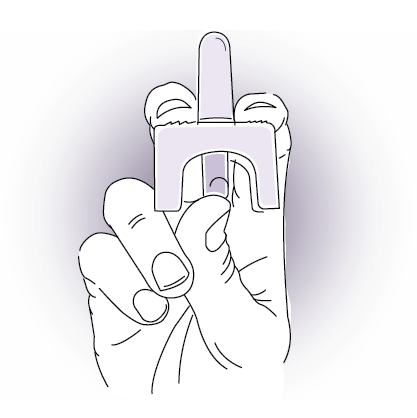 How To Use And How To Give Nayzilam Midazolam Nasal Spray Civ
How To Use And How To Give Nayzilam Midazolam Nasal Spray Civ
About NAYZILAM NAYZILAM midazolam nasal spray CIV is a benzodiazepine indicated for the acute treatment of intermittent stereotypic episodes.
Nayzilam package insert. Do not test or prime before use. In the US Nayzilam is indicated for the acute treatment of intermittent stereotypic episodes of frequent seizure activity ie seizure clusters acute repetitive seizures that are distinct from a patients usual seizure pattern in patients with epilepsy 12 years of age and older. Nayzilam is indicated for patients 12 years of age and older.
Nayzilam in pediatric patients less than 12 years of age have not been established 1. Hier sollte eine Beschreibung angezeigt werden diese Seite lässt dies jedoch nicht zu. Do not test or prime the spray before use.
Excursions permitted to 15C to 30C 59F. Nayzilam and Valtoco are benzodiazepines indicated for the acute treatment of intermittent stereotypic episodes of frequent seizure activity ie seizure clusters acute repetitive seizures that are distinct from a patients usual seizure pattern in patients with epilepsy. Selling or giving away this medicine may harm others and is against the.
Brophy GM Bell R Claassen J. Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Packaging Presentation Kit Components Capsules or Sachets Number of Doses per Kit NDC NumbersKit Components NDC Number Kit 160 mg Level 8.
And it should not take the place of a persons usual seizure medication. Nayzilam package insert. NAYZILAM is contraindicated in patients with acute narrow-angle glaucoma.
Remove the midazolam spray from the package only when you are ready to use it. Valeant Pharmaceuticals North America LLC. Do not open the blister packaging until ready to use.
Nayzilam is supplied in boxes of 2 nasal spray units NDC 50474-500-15 each contained within an individual blister pack. All three agents carry the same contraindication of. Lastly midazolam intranasal Nayzilam is indicated for acute treatment of intermittent stereotypic episodes of frequent seizure activity ie seizure clusters acute repetitive seizures that are distinct from usual seizure pattern in epilepsy participants 12 years of age or older.
SK Life Science Inc. Nayzilam midazolam is from a group of drugs called benzodiazepines. Do not use if the nasal spray unit appears damaged.
Prior approval is required to ensure the safe clinically appropriate and cost effective use of Nayzilam while maintaining optimal therapeutic outcomes. Years of age or older. It is NOT approved as a seizure medicine to be used on a daily basis.
13 Each box contains two doses. Nayzilam nasal spray is a prescription medicine used short term to treat seizure clusters also called acute repetitive seizures in adults and children at least 12 years old. Nayzilam package insert from UCB Our Mission The mission of the Epilepsy Foundation is to lead the fight to overcome the challenges of living with epilepsy and to accelerate therapies to stop seizures find cures and save lives.
It is very good at stopping seizures quickly when used intermittently or as needed for specific situations. Doing so will make you lose the dose. Gently insert the tip of the spray.
It is anticipated that NAYZILAM prescriptions will cost commercial patients 40 per box. For detailed information on NAYZILAMs safety profile including important warnings and precautions please refer to the Package Insert. Store at controlled room temperature 20C to 25C 68F to 77F.
1 With the NAYZILAM Savings Card. Keep in a safe place to prevent misuse and abuse. BLA 761143 Page 10 Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.