Any cranial electrical stimulation CES device used in the home setting. A chronic pain condition depression anxiety insomnia and posttraumatic stress disorder PTSD Interventions.
 Pdf Cranial Electrotherapy Stimulation For The Treatment Of Depression
Pdf Cranial Electrotherapy Stimulation For The Treatment Of Depression
CES devices are FDA-cleared to treat depression anxiety and insomnia and they can be used in both the home and clinical settings.
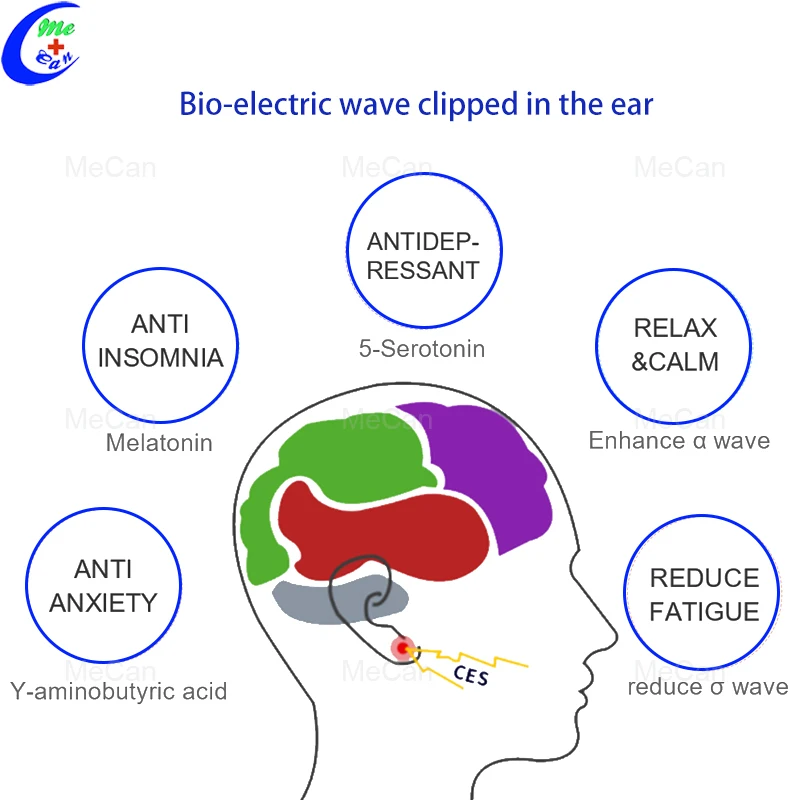
Cranial electrical stimulation for anxiety and depression. Cranial electrotherapy stimulation CES also known as transcranial electrostimulation electrosleep therapy and electronarcosis is a Food and Drug Administration FDA-approved non-invasive treatment for insomnia anxiety and depression. CES significantly decreases anxiety and comorbid depression. The Effectiveness and Risks of Cranial Electrical Stimulation for the Treatment of Pain Depression Anxiety PTSD and Insomnia.
Adult patients with one or more of the following conditions. Journal of Affective Disorders 164171-177 2014. Cranial electrotherapy stimulation CES is a safe modulation of brain activity for treating depression anxiety insomnia and pain.
Analysis of covariance revealed a significant difference between the active CES group and the sham CES group on anxiety p0001 d094 and on depression p0001 d078 from baseline to endpoint of study in favor of the active CES group. Usual care including appropriate known treatments. Using CES during a therapy session decreases anxiety and usually improves the patients ability to share prob-lems concerns and worries with the therapist as well as to respond to the therapists questions more effectively.
Pain severity use of opioid analgesic. Purpose To review evidence about the effectiveness and harms of CES for patients with chronic painful conditions depression anxiety PTSD and insomnia. Posted Sep 27 2018 Cranial electrotherapy stimulation CES has.
However there are no published studies in patients with advanced cancer ACPs. Anecdotal reports from psychiatrists and other mental. Alpha-Stim electrotherapy device is proven effective and safe for pain management and treatment of anxiety insomnia and depression.
This report is based on research conducted by the Evidence- based Synthesis Program ESP Center located at the West Los Angeles VA Medical Center Los Angeles CA. 104 Cranial electrical stimulation for the treatment of insomnia anxiety and depression symptoms in adults Jesús A. Cranial Electrotherapy Stimulation During Psychotherapy Sessions CES may also be used during psychotherapy sessions.
Moo-Estrella and María F. VA ESP Project 05-226. Barclay TH Barclay RD.
Cranial Electrotherapy Stimulation CES for Anxiety CES is FDA-approved for the treatment of anxiety depression and insomnia. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression. Subjects reported no adverse events during the study.
Cranial electrical stimulation CES is increasing in popularity as a treatment yet of uncertain clinical benefit. Cranial electrotherapy stimulation is a prescriptive medical device that delivers a mild form of electrical stimulation to the brain for the treatment of anxiety depression and insomnia. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression.
Cranial electrical stimulation CES is increasing in popularity as a treatment yet of uncertain clinical benefit. Presented at the American Psychological Association National Conference Honolulu HI July 2013. To review evidence about the effectiveness and harms of CES for patients with chronic painful conditions depression anxiety PTSD and insomnia.
The US Food and Drug Administration FDA has approved a cranial electrotherapy stimulator Cervella Innovative Neurological Devices for. Cranial electrotherapy stimulation is a prescriptive medical device that delivers a mild form of electrical stimulation to the brain for the treatment of anxiety depression and insomnia. Cranial electrotherapy stimulation CES is a safe modulation of brain activity for treating depression anxiety insomnia and pain.
However there are no published studies in patients with advanced cancer ACPs.



