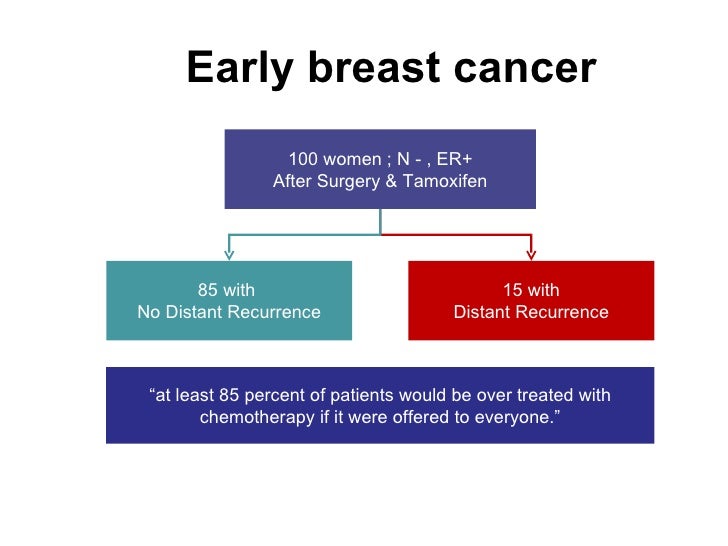
Keep in mind that these tests are usually used for early stage cancers and testing isnt needed in all cases. These studies included a total of 561188 patients who were subjected to genomic testing.
 Biomarkers And Commercial Strategy Comparing The Three Leading Breast Cancer Panels Amplion
Biomarkers And Commercial Strategy Comparing The Three Leading Breast Cancer Panels Amplion
1 The study explored the use of the MammaPrint 70-gene test to guide treatment decisions in.

Mammaprint vs oncotype. Data presented in Miami Breast conference in 2014 showed that the concordance between MammaPrint risk groups and Oncotype DX categories was 857 within the patients classified as high risk by Oncotype DX and 381 within the patients classified as high risk by MammaPrint. The Oncotype DX Breast Recurrence Score Test is used in two ways. Eight had intermediate RS with four high risk by MammaPrint.
Two had high risk both by RS and MammaPrint. Oncotype DX is only. Der MammaPrint-Test untersucht 70 Gene die ebenfalls Rückschlüsse auf das Rückfallrisiko zulassen.
In another comparative study Nunes and colleagues tested both gene signatures in 29 patients. More tests are in development. Oncotype gives a graded scale of risk broken up into low intermediate and high risk categories whereas MammaPrint is either low or high risk.
The Genomic Health Incs central testing laboratory has been approved by the US Clinical. The Objective of our study was to investigate the concordance of patient results from a single university centre tested with the 21-gene recurrence score assay Oncotype DX ODX when compared to the 70-gene signature Mammaprint MP the 80 gene signature of BluePrint BP and TargetPrint TP. Oncotype is applicable only to estrogen receptor ERpositive tumors.
To help doctors figure out a persons risk of early-stage estrogen-receptor-positive breast cancer coming back in a part of the body away from the breast distant recurrence The results of the Oncotype DX Breast Recurrence Score Test combined with other features of the. Some of the multigene assays that are currently available for early-stage breast cancers include the Oncotype DX Genomic Health Inc MammaPrint Agendia BV Prosigna PAM50 NanoString Technologies Inc and EndoPredict Myriad Genetics Inc. Evidence suggests that EndoPredict EPclin score Oncotype DX Breast Recurrence Score MammaPrint and Prosigna can predict the risk of distant recurrence in patients who have ER-positive HER2-negative early breast cancer.
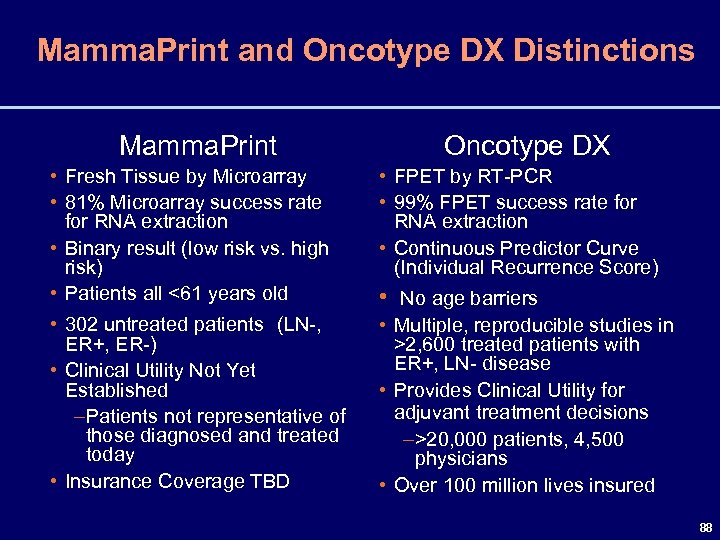
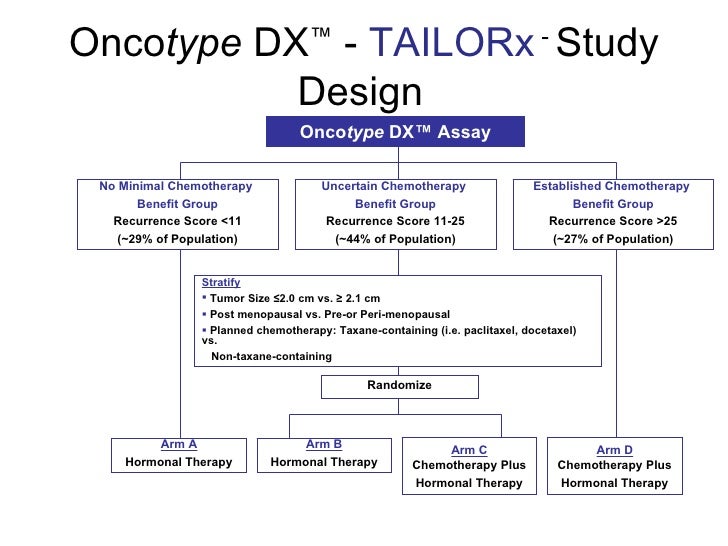
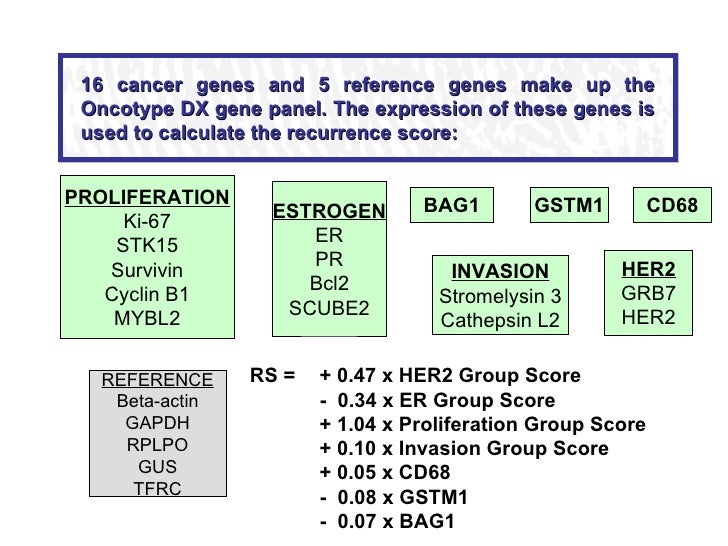
Purpose Risk of distant recurrence DR among women with estrogen receptor ER positive early breast cancer is the major determinant of recommendations for or against chemotherapy. The Oncotype DX test analyzes the activity of 21 genes and then calculates a recurrence score number between 0 and 100. The gene expression profiling assay OncotypeDx ODx prognosticates the risk of estrogen receptor positive ER breast cancer BC recurrence and assesses the likely benefit from adjuvant chemotherapy in addition to endocrine therapy.
Because of this strong research the Oncotype. Ist dies der Fall hätte sie von einer adjuvanten Chemotherapie sehr wahrscheinlich keinen größeren Nutzen als wenn sie diese Therapie nicht bekommen würde. The type of test thats used will depend on your situation.
The PAM50 risk of recurrence ROR score provides an alternative approach which also identifies. The MammaPrint test can now be added to the list of tests that help clinicians identify women who need chemotherapy and those who do not This focused update was initiated following the 2016 publication of findings from the MINDACT randomized phase III clinical trial. It is frequently estimated using the Oncotype DX recurrence score RS.
This evidence is strongest in the group with LN-negative disease which is likely to include patients with micrometastatic disease. The MammaPrint assay has received 510 k clearance by the FDA whereas Oncotype Dx has been exempt. Of the genomic tests used on breast cancer the Oncotype DX test has the most thorough data supporting its use to make treatment decisions.
The higher the score the greater the risk of recurrence of an invasive breast cancer. The Oncotype DX MammaPrint and Prosigna are examples of tests that look at different sets of breast cancer genes. The other molecular tests for breast cancer including MammaPrint Prosigna EndoPredict and Breast Cancer Index have not yet shown evidence of both capabilities she added.
MammaPrint can be used for both ER-positive and ER-negative tumors. The MammaPrint test made by Agendia is a genomic test that analyzes the activity of certain genes in early-stage breast cancer. Research suggests the MammaPrint test may eventually be widely used to help make treatment decisions based on the cancers risk of coming back recurrence within 10 years after diagnosis.
In total 540647 patients were tested by Oncotype DX 963 18614 by MammaPrint 33 1359 by. Mit seiner Hilfe lässt sich herausfinden ob eine bestimmte Patientin zur Niedrigrisikogruppe gehört.
 Table 2 From Title The 70 Gene Signature Mammaprint As A Guide For The Management Of Early Stage Breast Cancer Author Semantic Scholar
Table 2 From Title The 70 Gene Signature Mammaprint As A Guide For The Management Of Early Stage Breast Cancer Author Semantic Scholar
 Biomarkers And Commercial Strategy Comparing The Three Leading Breast Cancer Panels Amplion
Biomarkers And Commercial Strategy Comparing The Three Leading Breast Cancer Panels Amplion
 Oncotype Dx Coloprint For Prostate And Colon Cancer Calmerme
Oncotype Dx Coloprint For Prostate And Colon Cancer Calmerme
 Genomics In The Treatment Of Early Stage Breast
Genomics In The Treatment Of Early Stage Breast
 Testing To Avoid Treatment In Breast Cancer Conquer The Patient Voice
Testing To Avoid Treatment In Breast Cancer Conquer The Patient Voice
 The Comparison Of Two Clinically Relevant And Commercially Available Download Table
The Comparison Of Two Clinically Relevant And Commercially Available Download Table

 Risk Reclassification And Recurrence Rates For The Oncotype Dx And Download Table
Risk Reclassification And Recurrence Rates For The Oncotype Dx And Download Table
 Tests To Help With Breast Cancer Treatment Decisions Lymphfantastic
Tests To Help With Breast Cancer Treatment Decisions Lymphfantastic
 A Oncotype Dx And B Mammaprint Panel Scores For The 8 Breast Cancer Download Scientific Diagram
A Oncotype Dx And B Mammaprint Panel Scores For The 8 Breast Cancer Download Scientific Diagram
 Current Concepts In Breast Cancer Beyond Tnm Ppt Video Online Download
Current Concepts In Breast Cancer Beyond Tnm Ppt Video Online Download


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.