After reconstitution with 14 mL of Sterile Water for Injection USP the vial contains 150 mg of omalizumab per 12 mL of reconstituted solution for subcutaneous injection. The final admixed Soliris 5 mgmL infusion volume is.
 Study Treatment With Omalizumab Raises Tolerance In Patients With Severe Food Allergies Snacksafely Com
Study Treatment With Omalizumab Raises Tolerance In Patients With Severe Food Allergies Snacksafely Com
XOLAIR Omalizumab For Subcutaneous Use DESCRIPTION Xolair Omalizumab is a recombinant DNA-derived humanized IgG1κ monoclonal antibody that selectively binds to human immunoglobulin E IgE.

Omalizumab fda label. Healthcare Professional Information FDA Drug Safety Communication. The antibody has a molecular weight of approximately 149 kiloDaltons. XOLAIR for Injection Vial XOLAIR omalizumab for injection is a sterile white preservative free lyophilized powder in a single-dose vial.
Food and Drug Administration FDA has approved the companys supplemental Biologics License Application for Xolair omalizumab prefilled syringe for self-injection across all approved US. Xolair is produced by a Chinese hamster ovary cell. FDA Drug Safety Communication.
XOLAIR is indicated for patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and. Novartis receives FDA approval of Xolair omalizumab self-injection with prefilled syringe across all indications for appropriate patients. XOLAIR 75 mg prefilled syringe with a blue needle shield.
Omalizumab is a recombinant DNA-derived humanized IgG1κ monoclonal antibody that selectively binds to human immunoglobulin E IgE. Have a positive skin test or in vitro reactivity to a perennial aeroallergen. EAST HANOVER NJ Aug.
Xolair Omalizumab is indicated for adults and adolescents 12 years of age and above with moderate to severe persistent asthma who have a positive. The agency approved the companys supplemental Biologics License Application sBLA for Xolair. 1-888-835-2555 or FDA at 1 -800-FDA-1088 or.
FDA approves label changes for asthma drug Xolair omalizumab including describing slightly higher risk of. 16 rows Drug Name Active Ingredients Strength Dosage FormRoute Marketing Status TE. 1.
1 Xolair is the only FDA-approved biologic designed to target and. RHHBY today announced that the US. Pediatric Focused Safety Review for Xolair.
5 Dextrose in Water Injection USP. You should check the label on the carton that comes with the XOLAIR prefilled syringe to make sure that the dose is correct. If approved Xolair prefilled syringe would become available for.
Xolair Safety and Utilization Review. Or Ringers Injection USP to the infusion bag. XOLAIR prefilled syringes are available in 2 dose strengths.
Roche RHHBY announced that the FDA has approved the label expansion of asthma drug Xolair. FDA approves label changes for asthma drug Xolair omalizumab including describing slightly higher risk of. Xolair Clinical Pharmacology.
045 Sodium Chloride Injection USP. Current product labeling for the use of omalizumab in asthma notes that injection site reactions occurred in 45 of omalizumab treated patients compared with 43 of placebo treated patients. Amount equal volume of diluent to drug volume of 09 Sodium Chloride Injection USP.
And Symptoms inadequately controlled with inhaled corticosteroids. Xolair has been shown to decrease the incidence of asthma exacerbations in these patients. Label Xolair Xolair.
Xolair omalizumab for subcutaneous use is proven for patients with moderate to severe persistent asthma who meet all of the following criteria. Xolair is indicated for patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids. These instructions are to be used for both dose strengths.
Basel 13 April 2021 - Roche SIX. 13 2020 PRNewswire -- Novartis today announced that the US Food and Drug Administration FDA accepted the companys supplemental Biologics License Application sBLA for a new self-administration option for Xolair omalizumab across all approved US indications.
 Xolair Omalizumab Fda Package Insert Drug Facts Iodine Com
Xolair Omalizumab Fda Package Insert Drug Facts Iodine Com
 Xolair Label Updated With New Warnings Top Class Actions
Xolair Label Updated With New Warnings Top Class Actions
 Xolair Uses Heart Attacks Other Side Effects And Fda Actions
Xolair Uses Heart Attacks Other Side Effects And Fda Actions
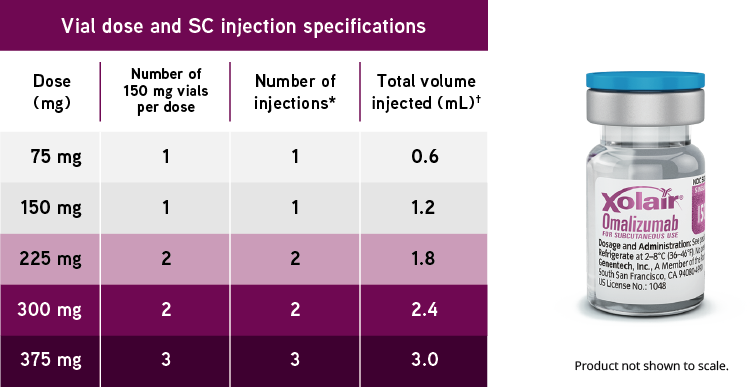 Xolair Omalizumab Dosing For Allergic Asthma Ciu Nasal Polyps
Xolair Omalizumab Dosing For Allergic Asthma Ciu Nasal Polyps
 Xolair Omalizumab Fda Package Insert Drug Facts Iodine Com
Xolair Omalizumab Fda Package Insert Drug Facts Iodine Com
 Allergy Notes Omalizumab Xolair Linked To Higher Risk Of Heart Attack Mini Stroke Tia Blood Clots Fda Changes The Label
Allergy Notes Omalizumab Xolair Linked To Higher Risk Of Heart Attack Mini Stroke Tia Blood Clots Fda Changes The Label
 Xolair Class Action Lawsuit Serving New York New Jersey
Xolair Class Action Lawsuit Serving New York New Jersey
 Xolair Cardiovascular Cerebrovascular Risk Parker Waichman Llp
Xolair Cardiovascular Cerebrovascular Risk Parker Waichman Llp
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2019 103976s5234lbl Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2019 103976s5234lbl Pdf
 Fda Adds Warning For Cardio Risks To Asthma Drug Xolair Allergic Living
Fda Adds Warning For Cardio Risks To Asthma Drug Xolair Allergic Living
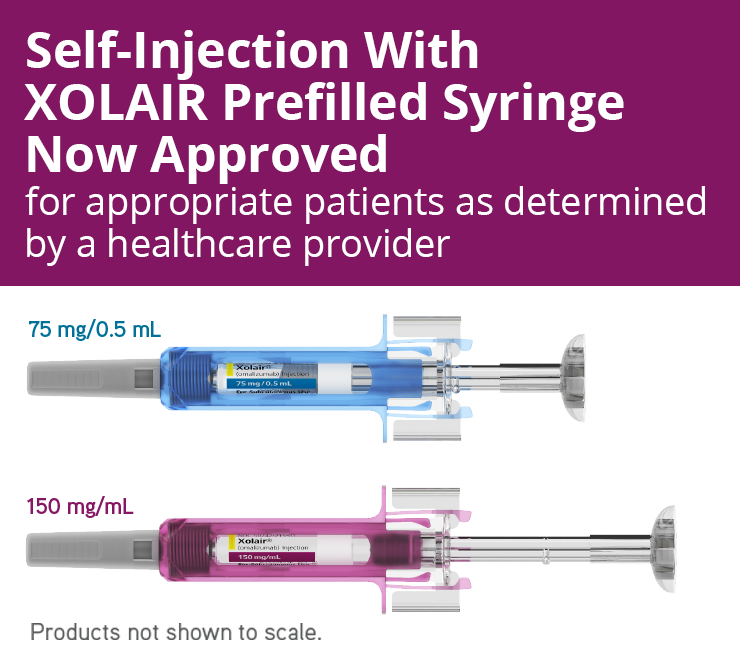 Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
Xolair Omalizumab Allergic Asthma Ciu Nasal Polyps Treatment
Https Www Fda Gov Media 124199 Download


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.